Thank you for your interest in CCO content. As a guest, please complete the following information fields. These data help ensure our continued delivery of impactful education.
Become a member (or login)? Member benefits include accreditation certificates, downloadable slides, and decision support tools.

Clinical Assistant Professor
Department of Clinical Pharmacy
University of Michigan College of Pharmacy
Outpatient Leukemia Pharmacy Specialist
Department of Pharmacy
Michigan Medicine
Ann Arbor, Michigan
Lydia Benitez, PharmD, BCOP, has no relevant financial relationships to report.

Clinical Pharmacy Specialist, Leukemia
Division of Pharmacy
University of Texas MD Anderson Cancer Center
Houston, Texas
Caitlin R. Rausch, PharmD, has no relevant financial relationships to report.
Key Takeaways:
In association with the 2022 Hematology Oncology Pharmacy Association Annual Conference, experts presented a live program titled “Applying Evidence and Expert Experience to Optimize Management and Overcome Clinical Challenges in CLL.” Here, Lydia Benitez, PharmD, BCOP, and Caitlin R. Rausch, PharmD, address topics that were raised by the audience during the question and answer session of this program.
Current initial treatment for newly diagnosed chronic lymphocytic leukemia (CLL) consists of Bruton tyrosine kinase (BTK) inhibitors or venetoclax in combination with a CD20-directed monoclonal antibody. Patient-specific factors such as comorbidities, ability to adhere to oral medications, and frequency of lab/clinic visits should be taken into consideration along with tumor burden, presence of del(17p)/TP53, and financial toxicity potential when selecting the most appropriate treatment option for patients (Figure).
Figure. Treatment algorithm for newly diagnosed CLL.
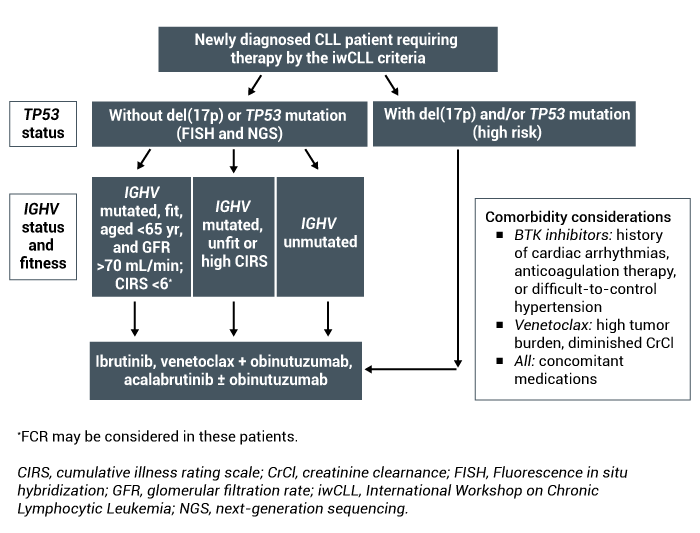
As patients move through treatment and potentially into the relapsed/refractory setting, it’s important to identify if a therapy change is warranted because of intolerance or because of progression. If a patient develops intolerance to a BTK inhibitor but is responding well to therapy, there is an opportunity to change within the drug class rather than changing therapeutic classes entirely. However, if a patient progresses on a BTK inhibitor, a therapeutic drug class change would be warranted.
Drug–drug interactions (DDI) are common with BTK inhibitors and venetoclax, particularly with agents metabolized by CYP3A4. Ensuring a comprehensive medication reconciliation is performed before treatment initiation and throughout treatment is crucial to prevent toxicities. Medications for other chronic disease states may need to be changed or chemotherapy may need to be dose adjusted to appropriately mitigate known DDIs.
Do you routinely assess CYP3A4 status before initiating therapy?
Lydia Benitez, PharmD, BCOP:
One of the most common things I do in my clinic is perform medication reconciliations for the patients and ensure I know every CYP3A4 agent they are receiving. If patients are receiving a CYP3A4 inhibitor, I explore potential alternatives to recommend. When the interaction involves drugs not managed by the oncology clinic, it can take some coordination to switch agents, such as contacting primary care physicians. In the case of venetoclax, I ensure the dose of venetoclax is reduced appropriately.
Caitlin R. Rausch, PharmD:
I completely agree. An initial medication reconciliation is critical before starting these patients on any therapy. Many times, the offending agent is one that is prescribed by another physician; as pharmacists, we are often investigating the potential for changing that interacting medication or dose reducing a medication depending on the context and indication. Certainly, it’s important to assess for DDIs at the start of therapy, but it’s equally important to assess throughout the entirety of treatment as well. This is often the explanation for an unexpected toxicity in the middle of therapy.
Lydia Benitez, PharmD, BCOP:
A pertinent CYP3A4 interaction right now is with the oral COVID-19 therapy nirmatrelvir/ritonavir. At my institution, we are primarily holding the BTK inhibitor or the BCL-2 inhibitor for the duration of the patient’s nirmatrelvir/ritonavir therapy rather than recommending an alternative COVID-19 treatment. We know we can safely interrupt the CLL treatment for short periods of time for things like this.
Caitlin R. Rausch, PharmD
I agree. There is certainly a precedent to hold the BTK inhibitors because of the recommendation to do so when a patient is undergoing surgery. We follow a similar practice of holding the medication for COVID-19 treatment with nirmatrelvir/ritonavir. The venetoclax can be a bit more complex. Despite having the option for dose adjustment, we also recommend to hold venetoclax during COVID-19 therapy; depending on the dose of venetoclax the patient is receiving, it can be difficult to determine the optimal dose of venetoclax to mitigate the DDI. Overall, holding the BTK and BCL-2 inhibitors is reasonable unless there’s a patient-specific reason not to.
The rate of atrial fibrillation is higher in patients receiving ibrutinib vs acalabrutinib in the ELEVATE trial. Do healthcare professionals differentiate between atrial fibrillation rates?
Lydia Benitez, PharmD, BCOP:
In the ELEVATE-RR trial, investigators found that the overall rates of atrial fibrillation were slightly higher in patients who received ibrutinib compared with acalabrutinib at 15.6% and 9.0%, respectively. The incidence was similar for grade ≥3 atrial fibrillation with occurrence rates of 4.5% in the acalabrutinib arm and 3.4% in the ibrutinib arm. There’s a numerical difference but I think the number needed to harm is very high.
Caitlin R. Rausch, PharmD:
Yes, I agree. The grade ≥3 rates were virtually the same between the two agents, and overall, the rate of atrial fibrillation was higher with ibrutinib. I think it’s important to be aware of that nuance, but if you have a patient with multiple risk factors for atrial fibrillation, I think it’s reasonable to choose acalabrutinib first.
What’s the real-world experience with cost-effective treatments and BTK inhibitors? What happens in countries that don’t have access to BTK inhibitors?
Lydia Benitez, PharmD, BCOP:
We both are very fortunate to practice in a privileged setting. It’s a great question of what to do if patients can’t afford or don’t have access to these medications. We have to acknowledge that there are patients in the United States—as well as other countries—who cannot benefit from these treatments due to the high cost and financial toxicity. It’s extremely important to acknowledge that fact and to try to do as much as possible by the way of awareness, even with trial design. For example, trials that are looking at BTK inhibitors in combination with BCL-2 inhibitors are great from a clinical standpoint. However, we also need to think about the applicability of these regimens to the real world and how much they drive the cost of care. Another thing to consider is clinically, does lowering measurable residual disease (MRD) make a difference regarding outcomes like overall survival? It’s a very provocative question that I don’t know if we can answer at this point, but I think it’s a great question to bring up.
Caitlin R. Rausch, PharmD:
Yes, I agree. This is a question that is not answered as often as it should be. We do have the luxury of being able to seek grants and other patient assistance through the manufacturer or other organizations. Despite having access to these resources, it is still difficult for some patients in the United States to obtain these agents and I’m sure it can be extremely difficult to get access to these drugs in other countries. Regarding MRD status, we learned in the 2-year follow-up from the CAPTIVATE study of ibrutinib vs placebo in those who had undetectable MRD, that there’s no benefit in continuing ibrutinib. The results indicate ibrutinib could be stopped after the 12 cycles of combination therapy, which would help mitigate some financial burden. Overall, we need more answers in this space.
Lydia Benitez, PharmD, BCOP:
I think a huge point of fixed-duration therapy is the potential mitigation of some financial toxicity. In the treatment-naive setting, I’ve encountered physicians and patients who are afraid to stop therapy after the fixed duration period is over. The argument against continuing therapy is you’re increasing financial toxicity. The CAPTIVATE study’s fixed-duration cohort saw good outcomes. The study hasn’t yet reached a median progression free-survival, so why increase the cost of care and risk of toxicities? It is nice that studies are looking at these things, as Dr. Rausch mentioned, but certainly much more is needed in this space.
What is your experience with BTK discontinuation rate because of toxicities, such as myalgias and gastrointestinal intolerance?
Caitlin R. Rausch, PharmD:
In the ELEVATE-RR trial, there were less myalgias overall with acalabrutinib vs ibrutinib. The real‑world study of tolerability with ibrutinib found that many patients didn’t tolerate ibrutinib because of arthralgia. Most often, we see discontinuation because of atrial fibrillation or other cardiac issues. It was interesting in the real-world study because arthralgias occurred at a very high rate in the frontline setting, but not in patients with relapsed disease. I’m not quite sure why myalgias had such a high incidence in this study when it really hasn’t been shown to be so significant in other settings. Anecdotally, I don’t notice a particular difference in toxicities that patients experience when they’re in the frontline vs the relapsed settings.
Lydia Benitez, PharmD, BCOP:
I don’t think there is a clinical rationale as to why we would see a difference in toxicity rates between the upfront and the relapsed setting. That difference is likely due to patient selection or patient reporting differences between the trials.
Caitlin R. Rausch, PharmD:
There is less overall diarrhea as well with acalabrutinib compared with ibrutinib. Acalabrutinib doesn’t hit EGFR whereas ibrutinib does; that’s the postulation for the reason for diarrhea with ibrutinib. The rates are pretty similar between the different BTK inhibitors for constipation.
Lydia Benitez, PharmD, BCOP:
With gastrointestinal toxicities, it’s important to acknowledge the drug effect but also to take the patient as a whole into consideration. Many of these patients have comorbidities that could be affecting their gastrointestinal tract. It’s important to address all of those aspects. There could be other comorbidities coming into play and the drug is a contributor to the problem but may not be the source. Drugs are usually thought to be the culprit, but as a pharmacist, sometimes you need to play detective.
Your Thoughts
What challenges have you encountered in treating patients with CLL? Share your experiences in the comment box below.

Contact Clinical Care Options
For customer support please email: customersupport@cealliance.com
Mailing Address
Clinical Care Options, LLC
12001 Sunrise Valley Drive
Suite 300
Reston, VA 20191
You are now leaving the CCO site. The new destination site may have different terms of use and privacy policy.